
Carbon electron configuration noble gas notation full#
The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. We know from the periodic table that copper is in group 11, so this agrees with the result of this electron configuration calculator.Electron configuration chart of all Elements is mentioned in the table below. Let us look at the electron configuration for helium, which is 1 s 2 1 4s^1 3 d 10 4 s 1.
They are helium, neon, argon, krypton, xenon, and radon. The elements that are found in the last column of the periodic table are an important group of elements called the noble gases. The remaining two electrons will go in the 2p orbital. Since 1s can only hold two electrons the next 2 electrons for C goes in the 2s orbital. 81 Noble gas A Group 8 element, 94 electron configuration of. In writing the electron configuration for carbon the first two electrons will go in the 1s orbital. 339 as essential element, 690t in human body, 77t Lewis structure of, 371 oxidation of.

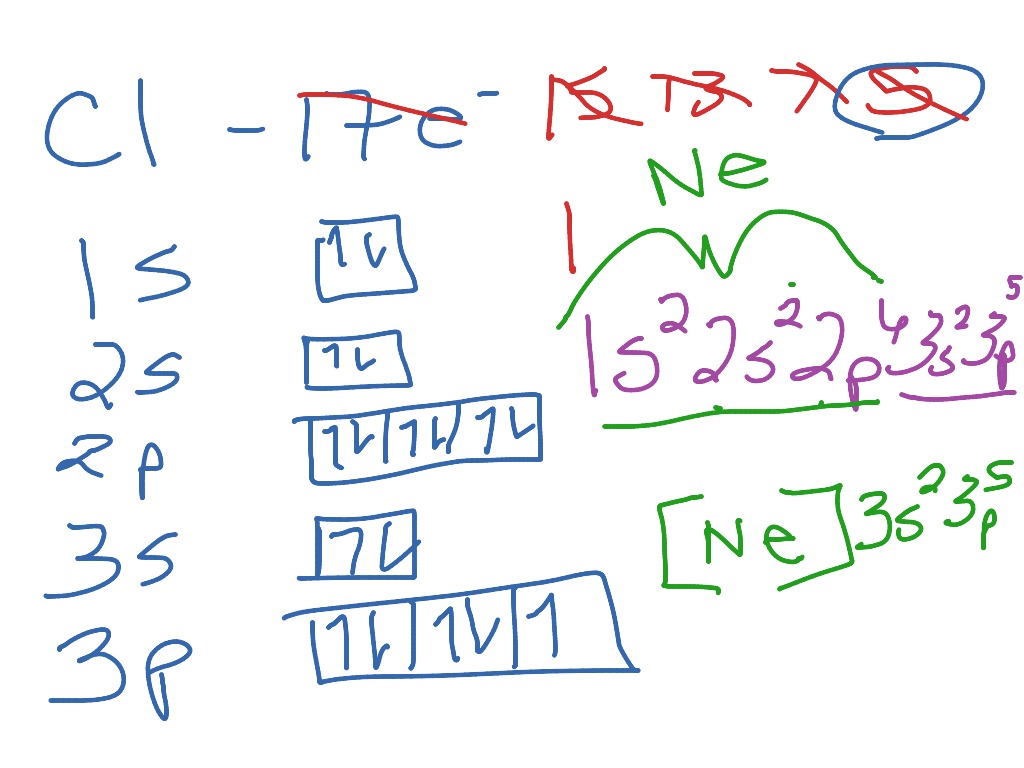
For instance, for the element aluminum we write We may NOT use any element in the brackets, only noble gases. The noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of the atom’s valence electrons. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating for the loss of or gain of electrons in their subsequent orbitals (we will examine those in the next section). Carbon is the sixth element with a total of 6 electrons. For example, magnesium (Mg) has the electron configuration 1s2, 2s2, 2p6, 3s2. The electron configuration is responsible for many physical and chemical properties of an element. In this method of writing electron configurations, the last noble gas before we get to the element of interest is the noble gas we put into the brackets. We can replace a chunk of an elements electron configuration with its noble gas equivalent. Write the complete electron-configuration notation, the noble-gas notation, and the orbital notation for the following elements: a. Ground state means that the atom has the lowest energy allowed.
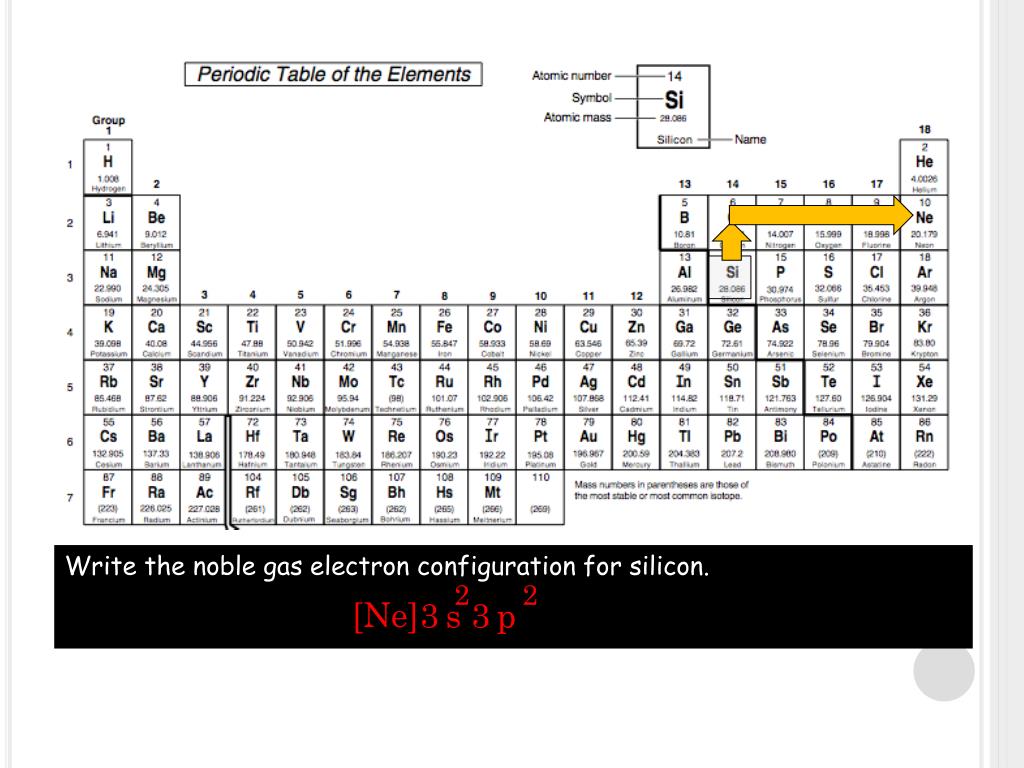
Question: Give the noble gas electron configuration of carbon (C).

Electron configuration notation provides us with information about the basic energy levels and sublevels that electrons occupy. Science Give the noble gas electron configuration of carbon (C).


 0 kommentar(er)
0 kommentar(er)
